Update on Notified Bodies
- 15 August 2022
- Posted by: Roger Gray
- Category: GLOBAL NEWS
No Comments
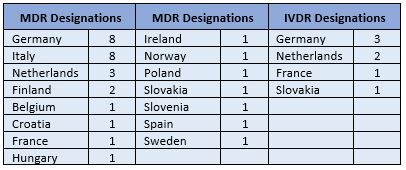
Several recent additions have been added to the NANDO databases for Notified Bodies (NBs) designated under the Medical Device Regulation (MDR, 2017/745) and In Vitro Diagnostics Regulation (IVDR, 2017/746).
The latest assessment bodies to be designated are Bureau Veritas Italia and the Certification Division of the Spanish Agency for Medicines and Healthcare Products (AEMPS).
32 NBs have now been designated for the MDR and 7 for the IVDR, with following breakdown by country: