FDA advises new fees for FY 2023
- 6 October 2022
- Posted by: inetika
- Category: GLOBAL NEWS
No Comments
The US Food and Drug Administration has now published its revised user fees for its fiscal year (FY) 2023, which starts on 1 October 2022.
Any submissions or applications arriving at the Agency on or after 1 October 2022 will need to have paid the new fees, which are:
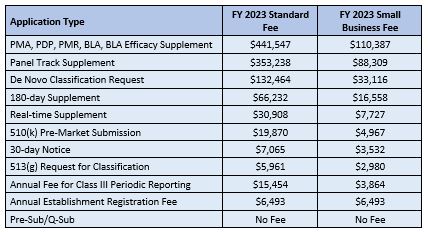
As can be seen from the above table, some of the fees have increased by substantial amounts since the previous year, including the 510(k) review fee, which has jumped over 50%.
Further details are available from here.

