FDA announces user fees for fiscal 2025
- 2 August 2024
- Posted by: inetika
- Category: GLOBAL NEWS
No Comments
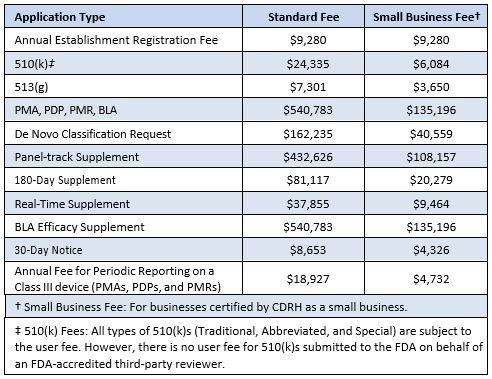
The US Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) has published the user fees applicable to submissions, etc for fiscal year 2025, running from 1 October 2024 to 30 September 2025. The new fees represent a 12% increase over the 2024 fees. Further details are available from the FDA website.
The 2025 fees are: